Case Study
Timeline
Although the Product Owner had made significant progress with the design, it was clear that the design and UX required further evolution before the FDA submission and Go To Market campaign. I was brought in as a consultant, and my initial task was to conduct a comprehensive Expert Review using custom Heuristics to pinpoint all observable issues.
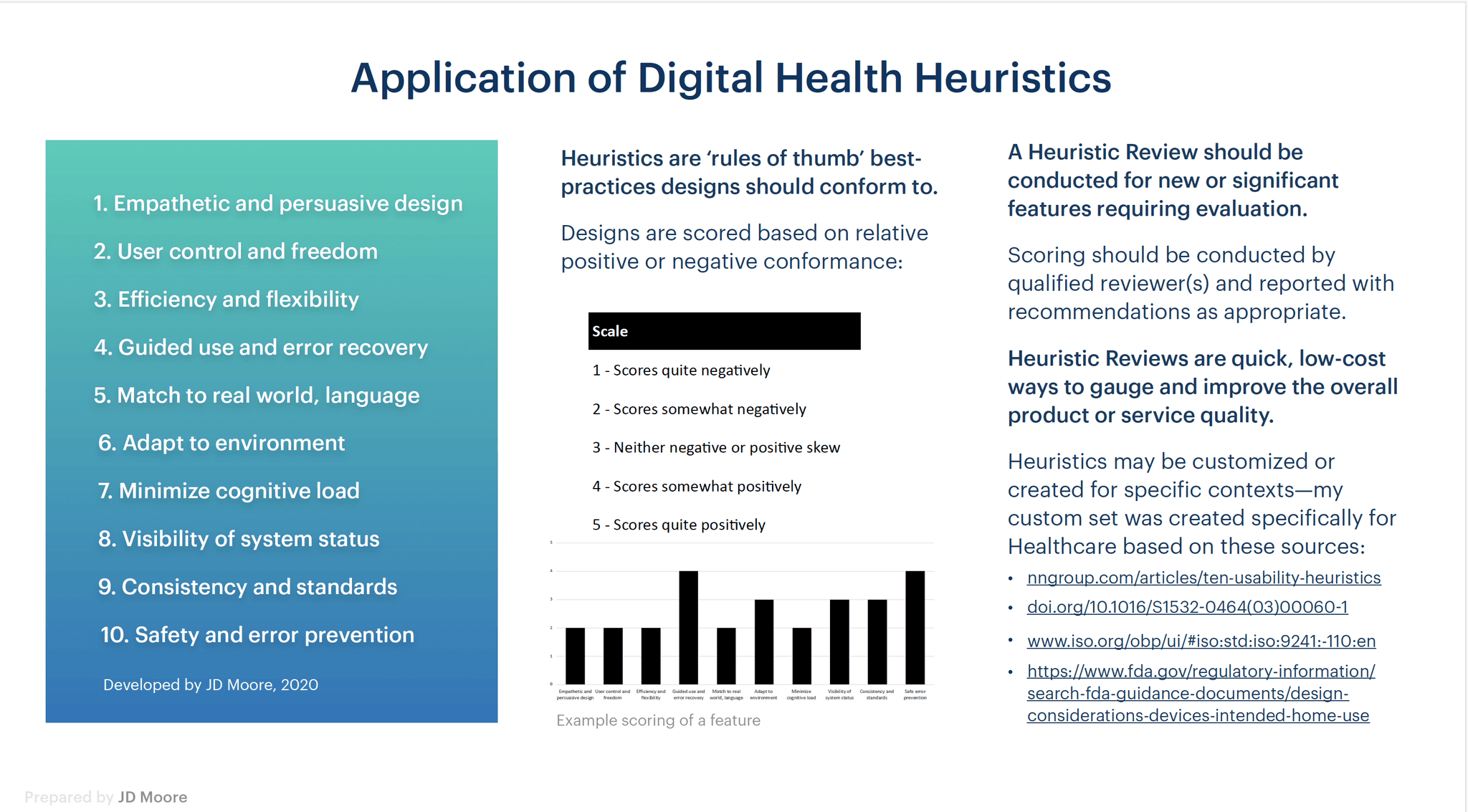
Once the initial Heuristic / Expert Review was conducted, I spent time with the Product Team, as well as Sales & Marketing, Development and Clinical to further identify ancillary issues and used a 'spider chart' Scorecard system to prioritize.
Although I am an accomplished UX professional, I have never claimed to be a world-class Visual Designer. I negotiated with the client to bring in one of my top-tier designers who could enhance ongoing efforts with Branding, Logo Design, Iconography, and collaborate on all UX aspects.
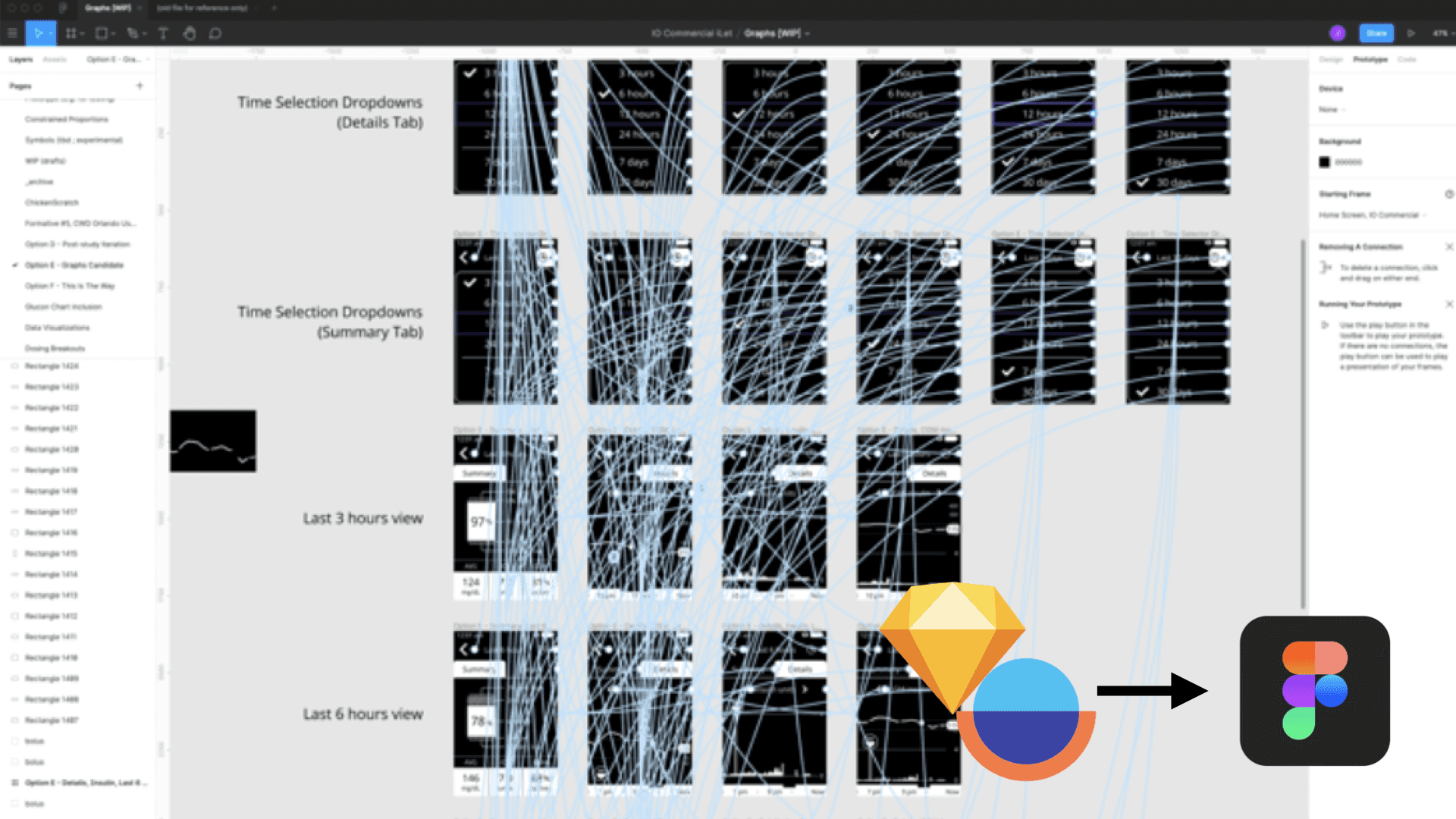
With the involvement of the soon-to-be first-ever full-time designer, we enhanced the Design Toolkit and streamlined the Design Process for an effective handover. Simultaneously, I continued to consult with the client to refine their Research Process, Prototyping, and reporting methods.
Due to challenges with testing the UI/UX in a heavily regulated environment, I built a new testing platform that allowed would-be Patients and their Caregivers to hold a similarly-sized device (mobile phone) in their hands, and interact with the actual device's digital interface in a remote—albeit more realistic—setting. This greatly expanded the reach of the participant pool and sped up iteration.
Once the enhanced in-house Design and Research functions were operational, we concluded the project by planning a forthcoming Mobile App and introducing comprehensive mapping tools (Journey Maps, Customer Value Propositions, Ecosystem Map) that defined a next-phase UX Strategy.
In May '23, the manufacturer announced FDA clearance
An improved Design Process and Toolkit was established, along with new Research platform including automated Consent forms
A custom set of Heuristics was developed specifically for at-home-use Medical Devices, incorporating industrial design, NN/g Usability principles, ISO standards, and FDA guidelines
By the end of the project, Design was fully operational with the first full-time Designer